
Fluorescence is one of the most fascinating and useful phenomena in modern physics — a light that emerges from matter itself, capable of revealing what the naked eye cannot see.
Today, fluorescence is widely used in science, medicine, industry, and environmental analysis, and has evolved into advanced analytical techniques such as Total Reflection X-ray Fluorescence (TXRF), developed by GNR Analytical Instruments Group, an Italian company with over 40 years of experience in materials analysis.
From a physical and chemical standpoint, fluorescence is a type of luminescence, i.e., the spontaneous emission of light from a substance after it has absorbed energy.
When a material is excited — for instance by ultraviolet radiation or X-rays — its electrons move to higher energy states. When they return to their initial state, the excess energy is released as photons: the fluorescent light we observe.
Fluorescence differs from phosphorescence in the duration of the emission.
“In fluorescence, the release of light occurs almost instantaneously (within nanoseconds), while in phosphorescence it can last for seconds, minutes, or even hours after the excitation source is removed”.
Real-world examples of fluorescence include:
- Minerals such as fluorite or calcite
- Biological fluids and protein markers in laboratory research
- Industrial materials, such as paints, pigments, and polymers containing optical brighteners
How fluorescence works: from physical principle to application
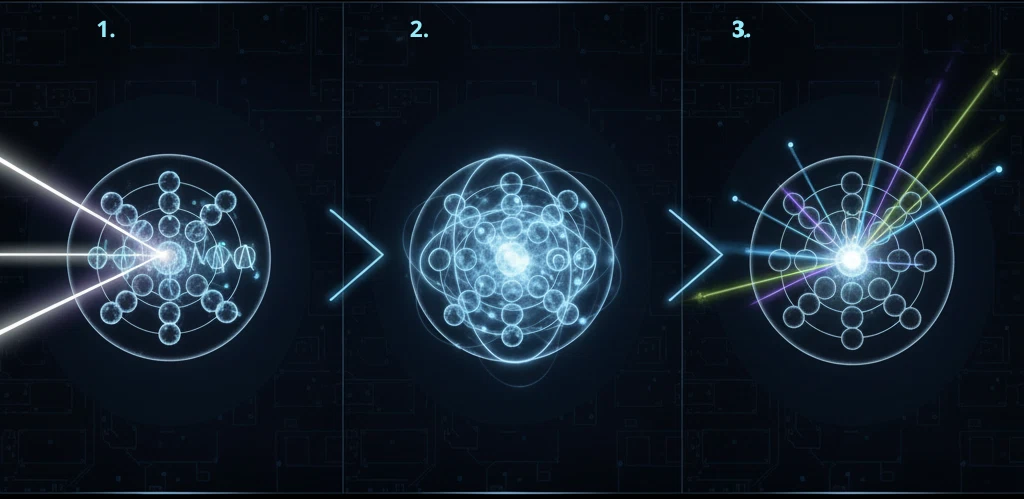
The fluorescence process follows an apparently simple, yet physically sophisticated and powerful mechanism.
It begins with the absorption of energy from an external source — ultraviolet light, high-intensity visible light, or, in advanced analytical techniques, X-rays.
This energy excites the sample’s electrons: in optical fluorescence, it typically involves outer or molecular orbitals, whereas in XRF (X-ray fluorescence) the excitation creates vacancies in inner electron shells.
In the following billionths of a second, the electrons return to their ground state, releasing the excess energy as photons — either visible light, ultraviolet light, or X-rays, depending on the process.
The energy and wavelength of the emitted photons depend on the specific atom or molecule involved. Each element possesses a unique spectral signature, a set of characteristic emission lines that distinguish it from all others.
This property makes fluorescence an extremely accurate and selective analytical method: by studying the emitted spectrum, scientists can identify and quantify the elements present in a sample with great precision, even at very low concentrations.
This simple yet powerful principle forms the basis of many modern analytical techniques — from biological microscopy to X-ray fluorescence (XRF) used for characterizing metals, alloys, and complex materials.
Types of fluorescence and main characteristics
The phenomenon of fluorescence can appear in different forms, depending on the excitation source and the application context:
- Natural fluorescence – Found in nature in certain minerals, marine organisms and plants, where the emitted light serves as a means of communication or protection.
- Induced fluorescence – Used in biology, medicine and chemistry to reveal phenomena invisible to the naked eye, often through fluorescent tracers or markers.
- X-ray fluorescence (XRF) – One of the most powerful analytical techniques for material characterization, based on the emission of characteristic X-ray photons by excited atoms.
This last type represents the bridge to the most advanced industrial applications and the innovative analytical solutions developed by GNR.
Where fluorescence is used: main fields of application
Thanks to its versatility and sensitivity, fluorescence is employed across a wide range of scientific and industrial sectors, supported by specific instruments and technologies that enhance its analytical potential.
- Biology and medicine – Fluorescence forms the basis of many biological imaging and molecular diagnostics techniques. Microscopes and optical spectrometers detect fluorescent markers that allow scientists to observe cells, tissues and proteins in real time, supporting biomedical research and non-invasive diagnostics.
- Chemistry and environmental analysis – In chemical and environmental laboratories, TXRF (Total Reflection X-Ray Fluorescence) spectrometers are used to detect and quantify trace elements such as heavy metals, organic contaminants and pollutants. This technique achieves extremely low limits of detection (down to ng/g) using minimal sample amounts and without complex preparation steps.
- Geology and mineralogy – Fluorescence helps determine the composition of minerals and rocks, revealing elements such as iron, copper, titanium or manganese. X-ray fluorescence (XRF) allows precise identification and quantification of the chemical elements in solid or powdered samples, supporting petrographic and mineral classification.
- Metallurgy and materials industry – In this sector, XRF is a key technique for elemental analysis and quality control. Laboratory XRF spectrometers determine the chemical composition of ferrous and non-ferrous alloys, monitor production processes and ensure that materials meet quality standards.
X-ray fluorescence: principles and advantages
X-ray fluorescence (XRF) is an analytical method based on the excitation of atoms by an X-ray beam. When inner-shell electrons are ejected, outer electrons fall into their place, emitting characteristic X-ray photons unique to each element.
👉 Discover more about X-ray fluorescence by GNR
Main advantages of XRF
- Non-destructive technique – No invasive preparation and no alteration of the sample’s structure, making it ideal for analysing valuable or unique materials.
- High accuracy and analytical sensitivity – XRF systems provide precise results with detection limits reaching a few ng/g, maintaining accuracy even in complex or multi-component matrices.
- Applicable to solids, liquids and powders – The technique is highly versatile, suitable for metals, alloys, solutions, environmental dust and powders.
- Simultaneous multi-element analysis – In a single measurement, XRF can identify and quantify multiple elements, reducing testing time and operational costs.
GNR instruments for X-ray fluorescence: experience and innovation
For over forty years, GNR Analytical Instruments Group has been designing and manufacturing high-performance scientific instruments for materials analysis, combining research, precision and reliability.
With deep expertise in advanced analytical techniques — from optical emission spectrometry to X-ray diffraction and X-ray fluorescence — GNR has become an international reference point for laboratories, industries and research centers.
X-ray fluorescence (XRF) represents one of the company’s core areas of excellence. GNR systems enable qualitative and quantitative analysis on a wide range of materials, delivering accurate results in a short time and with minimal sample preparation.
Thanks to flexible configurations and dedicated software, these instruments are designed to meet the needs of quality control, applied research and new materials development.
XRF technologies are widely used in several fields:
- Metallurgy and materials industry – Composition analysis of ferrous and non-ferrous alloys.
- Environment and safety – Monitoring of pollutants, dust and contaminants in solid or liquid matrices.
- Chemical and pharmaceutical sectors – Determination of elements in complex compounds and finished products.
- Scientific and academic research – Characterization of advanced materials, nanostructures and multi-matrix samples.
Each instrument is engineered and assembled in Italy, in full compliance with international quality and metrological standards, and supported by a specialized technical team that assists customers in every phase — from configuration to calibration.
🔗 Visit GNR’s official website
📂 Explore all instrument categories
Horizon: TXRF benchtop spectrometer for high-precision multi-element analysis
Horizon is a new-generation benchtop spectrometer designed for qualitative and quantitative multi-element analysis through Total Reflection X-Ray Fluorescence (TXRF).
This technique, part of the EDXRF (Energy Dispersive X-Ray Fluorescence) family, relies on a narrow-angle X-ray beam hitting a thin film of the sample deposited on a reflective support. The extremely low incidence angle enhances the total reflection effect, amplifying the fluorescent signal and minimizing background noise.
The result is the simultaneous analysis of multiple elements, from sodium (Na) to plutonium (Pu), with limits of detection (LOD) as low as a few ng/g or below. TXRF provides key advantages: no matrix effects, no calibration curves dependent on matrix composition, and minimal sample quantities, often less than one microliter.
Horizon integrates the latest-generation components, ensuring accuracy, reproducibility and ease of use. Its compact design and intuitive software allow for quick, reliable results even in non-specialized laboratories.
It is particularly suited for:
- Environmental analysis – Detection of heavy metals in water and airborne particulate matter.
- Chemical and pharmaceutical applications – Purity control and micro-trace determination in solutions.
- Advanced materials and nanoscience – High-sensitivity analysis of innovative materials and thin films.
In summary, Horizon represents the state of the art in benchtop TXRF, combining high performance, cost efficiency and operational reliability.
TX 2000: laboratory TXRF spectrometer for complex applications
TX 2000 is a reference-grade laboratory spectrometer for quantitative multi-element trace and ultra-trace analysis using Total Reflection X-Ray Fluorescence (TXRF). Designed for advanced research and environmental applications, it combines power, sensitivity and flexibility in a single instrument.
The TXRF technique uses an X-ray beam that strikes the sample at a very low incidence angle, enhancing the fluorescent signal intensity. The sample, deposited as a thin film, is analyzed with exceptional sensitivity, eliminating matrix effects and allowing direct, accurate quantification without matrix-dependent calibration curves.
The TX 2000 features an automatic primary beam switch system (MoKa, WLa/Lb and bremsstrahlung 33 keV), a Peltier-cooled Silicon Drift Detector (SDD) with energy resolution <133 eV, and a high-power X-ray tube.
This configuration ensures:
- Detection limits below ng/g, ideal for detecting environmental contaminants and trace elements.
- Selectable monochromatic energy, optimized for the elements of interest.
- Stepper motors with optical encoders, ensuring precise angular control.
- Microanalysis capability and helium flow option to improve sensitivity for light elements.
With these features, TX 2000 is ideally suited for:
- Environmental and safety monitoring – Detection of metallic and inorganic pollutants in air, water and soil.
- Industrial and chemical laboratories – Purity and composition control of reagents and products.
- Energy and nuclear sectors – Trace analysis of critical elements and radioactive materials.
- Scientific research – Study of alloys, semiconductors and nanomaterials.
TX 2000 is the optimal solution for laboratories requiring maximum sensitivity and precision, combining research-grade performance with outstanding reliability.
Fields of application of X-ray fluorescence
Thanks to its ability to perform rapid, non-destructive, multi-element analyses, X-ray fluorescence (XRF) has become a key technology across numerous industrial and scientific domains.
XRF instruments are used by companies, laboratories and research institutions that need precise data on the chemical composition of materials.
- Metallurgy and alloys – Used by foundries, steelworks, extrusion plants and surface treatment companies to verify alloy composition and ensure compliance with production standards.
🔗 Learn more about metal analysis with Optical Emission Spectrometry (OES) - Chemical industry – Utilized by formulation laboratories and process plants for detecting elements in complex matrices and monitoring product purity.
- Environment and safety – Applied in environmental testing labs and regulatory agencies to detect heavy metals, pollutants and particulate matter in air, water and soil.
- Energy and nuclear – Essential for measuring trace elements and contamination in combustion residues, containment systems and renewable energy materials such as solar cells and batteries.
- Scientific and academic research – Employed by universities and R&D centers for the characterization of advanced materials, nanocomposites, ceramics and functional coatings.
In all these contexts, X-ray fluorescence stands out as a reliable, precise and sustainable analytical method, capable of reducing testing time, operational costs and reagent consumption compared to more invasive techniques.
FAQ
Fluorescence is a physical phenomenon in which a substance emits electromagnetic radiation after absorbing energy. At the atomic level, it results from electronic transitions between energy levels: an excited electron releases the excess energy as a photon when it returns to its ground state.
When atoms or molecules are excited by radiation (UV, visible or X-rays), their electrons move to higher energy states. As they return to their stable configuration, they emit light with a wavelength characteristic of the element or molecule involved.
Both belong to the family of luminescence phenomena, but they differ in the duration of emission. In fluorescence, light is emitted almost instantaneously (in nanoseconds), while in phosphorescence electrons remain trapped in metastable states, continuing to emit light even after the excitation source is removed.
Fluorescence techniques are widely applied in biology and medicine (cell markers, diagnostics), chemistry and environmental science (contaminant detection), geology and mineralogy (mineral identification), and materials science (analysis of alloys, ceramics and coatings).
X-ray fluorescence (XRF) is an analytical technique based on atomic excitation by an X-ray beam. The emission of characteristic X-ray photons from inner-shell electron transitions enables the identification and quantification of elements present in a sample.
XRF is non-destructive, multi-elemental, and applicable to solids, liquids and powders. It offers high sensitivity (detection limits down to ng/g) and short analysis times, often requiring minimal or no sample preparation.
GNR develops advanced spectrometers based on TXRF (Total Reflection X-Ray Fluorescence) technology, designed for high-resolution multi-element analysis, with detection limits down to ng/g and exceptional signal stability over time.
XRF technologies are used in metallurgy and alloy production, environmental and safety analysis, chemical and pharmaceutical industries, energy and nuclear applications, and in research laboratories and universities for the characterization of materials and the development of innovative technologies.

